
Chemistry, 21.08.2019 01:30 mjlchance367
An electrochemical cell at 25°c is composed of pure copper and pure lead solutions immersed in their respective ionis. for a 0.6 m concentration of cu2+, the lead electrode is oxidized yielding potential of 0.507 v. a cell a) calculate the concentration of pb2+ b) suppose the lead electrode is reduced, in that case what would be the concentration of pb2 what does this answer tell you?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:10, ChaseRussell24
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3


Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1
You know the right answer?
An electrochemical cell at 25°c is composed of pure copper and pure lead solutions immersed in their...
Questions in other subjects:




Mathematics, 03.03.2020 04:23


Mathematics, 03.03.2020 04:24




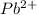 is, 0.0337 M
is, 0.0337 M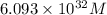
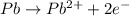
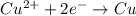
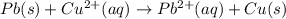
![E^o_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0183/3095/d356c.png)
![E^o_{[Cu^{2+}/Cu]}=+0.34V](/tpl/images/0183/3095/0d165.png)
![E^o=E^o_{[Cu^{2+}/Cu]}-E^o_{[Pb^{2+}/Pb]}](/tpl/images/0183/3095/2597c.png)
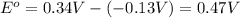
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Pb^{2+}]}{[Cu^{2+}]}](/tpl/images/0183/3095/2e714.png)
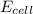 = 0.507 V
= 0.507 V![0.507=0.47-\frac{0.0592}{2}\log \frac{[Pb^{2+}]}{(0.6)}](/tpl/images/0183/3095/33110.png)
![[Pb^{2+}]=0.0337M](/tpl/images/0183/3095/42065.png)
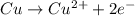
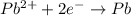
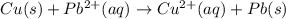
![E^o=E^o_{[Pb^{2+}/Pb]}-E^o_{[Cu^{2+}/Cu]}](/tpl/images/0183/3095/264d0.png)
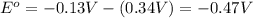
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Cu^{2+}]}{[Pb^{2+}]}](/tpl/images/0183/3095/9bec7.png)
![0.507=-0.47-\frac{0.0592}{2}\log \frac{(0.6)}{[Pb^{2+}]}](/tpl/images/0183/3095/15aa8.png)
![[Pb^{2+}]=6.093\times 10^{32}M](/tpl/images/0183/3095/e8ea8.png)



