
Consider the following reaction where kc = 9.52×10-2 at 350 k. ch4(g) + ccl4(g) 2ch2cl2(g)a reaction mixture was found to contain 2.18×10-2 moles of ch4(g), 3.79×10-2 moles of ccl4(g) and 1.09×10-2 moles of ch2cl2(g), in a 1.00 liter container. is the reaction at equilibrium? if not, what direction must it run in order to reach equilibrium? the reaction quotient, qc, equals .the reactiona. must run in the forward direction to reach equilibrium. b. must run in the reverse direction to reach equilibrium. c. is at equilibrium.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, dsawnstevensonp9xhnr
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 3

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 21.06.2019 19:30, angelinararr5783
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1
You know the right answer?
Consider the following reaction where kc = 9.52×10-2 at 350 k. ch4(g) + ccl4(g) 2ch2cl2(g)a reaction...
Questions in other subjects:

Mathematics, 08.03.2021 20:20

Mathematics, 08.03.2021 20:20

Mathematics, 08.03.2021 20:20

Mathematics, 08.03.2021 20:20


Mathematics, 08.03.2021 20:20

Social Studies, 08.03.2021 20:20



Mathematics, 08.03.2021 20:20

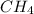 =
= 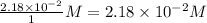
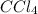 =
= 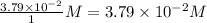
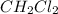 =
= 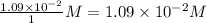
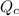 , for this reaction =
, for this reaction = ![\frac{[CH_{2}Cl_{2}]^{2}}{[CH_{4}][CCl_{4}]}](/tpl/images/0183/1287/5dd97.png)
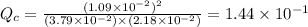
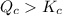 therefore reaction must run in reverse direction to reduce
therefore reaction must run in reverse direction to reduce 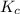 .
.

