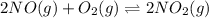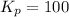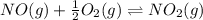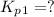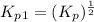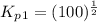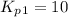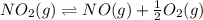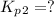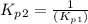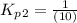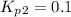Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1


Chemistry, 23.06.2019 09:30, sharmadaman641
What is the best describtion of the side of the moon that faces earth?
Answers: 2
You know the right answer?
Find the equilibrium constants, kp, for the following equilibria, (i) no(g) + ½ o2(g) ⇄ no2(g), kp =...
Questions in other subjects:


Mathematics, 18.11.2020 20:00

English, 18.11.2020 20:00

History, 18.11.2020 20:00

History, 18.11.2020 20:00



Mathematics, 18.11.2020 20:00

English, 18.11.2020 20:00