

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, ladypink94
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3
You know the right answer?
The complete combustion of octane, c8h18, a component of gasoline, proceeds as follows: 2c8h18(l)+2...
Questions in other subjects:

Mathematics, 19.07.2019 12:00

Mathematics, 19.07.2019 12:00


Chemistry, 19.07.2019 12:00


Biology, 19.07.2019 12:00

English, 19.07.2019 12:00

Chemistry, 19.07.2019 12:00

Physics, 19.07.2019 12:00

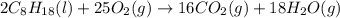
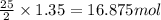 of oxygen are needed.
of oxygen are needed.


