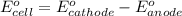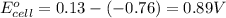Chemistry, 20.08.2019 05:10 strongl3219
Agalvanic (voltaic) cell consists of an electrode composed of zinc in a 1.0 m zinc ion solution and another electrode composed of copper in a 1.0 m copper(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25 °c. standard reduction potentials can be found here.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

You know the right answer?
Agalvanic (voltaic) cell consists of an electrode composed of zinc in a 1.0 m zinc ion solution and...
Questions in other subjects:

Mathematics, 27.10.2020 14:40



Physics, 27.10.2020 14:40



Arts, 27.10.2020 14:40

German, 27.10.2020 14:40

English, 27.10.2020 14:40

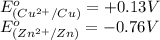
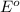 potential will always get reduced and will undergo reduction reaction. Here, copper will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, copper will undergo reduction reaction will get reduced.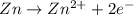
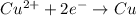
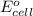 of the reaction, we use the equation:
of the reaction, we use the equation: