
Chemistry, 20.08.2019 02:30 notseansafe
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium constant for this reaction will
a. increase if the temperature is decreased.
b. decrease with increasing temperature.
c. increase with increasing temperature.
d. increase at some pressures and decrease at other pressures.
e. not change if the temperature is increased.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3


You know the right answer?
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium con...
Questions in other subjects:

History, 01.02.2021 17:30


Mathematics, 01.02.2021 17:30

Mathematics, 01.02.2021 17:30



Physics, 01.02.2021 17:30


Mathematics, 01.02.2021 17:30

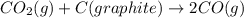
![K_{c} = \frac{[CO_{2}]}{[CO]^{2}}](/tpl/images/0180/4423/2161b.png)


