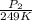The vapor pressure of liquid iodomethane, ch3i, is 40.0 mm hg at 249 k. a sample of ch3i is placed in a closed, evacuated container of constant volume at a temperature of 404 k. it is found that all of the ch3i is in the vapor phase and that the pressure is 72.0 mm hg. if the temperature in the container is reduced to 249 k, which of the following statements are correct
choose all that apply
a. the pressure in the container will be 104 mm hg.
b. no condensation will occur.
c. some of the vapor initially present will condense.
d. only pentane vapor will be present.
e. liquid pentane will be present.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
The vapor pressure of liquid iodomethane, ch3i, is 40.0 mm hg at 249 k. a sample of ch3i is placed i...
Questions in other subjects:

Geography, 25.07.2019 07:30

Biology, 25.07.2019 07:30

Mathematics, 25.07.2019 07:30






English, 25.07.2019 07:30

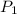 = 72.0 mm Hg,
= 72.0 mm Hg, 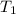 = 404 K
= 404 K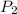 = ? ,
= ? , 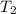 = 249 K
= 249 K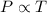 (at constant volume)
(at constant volume)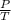 = k
= k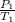 =
= 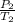
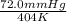 =
=