
Chemistry, 19.08.2019 23:20 regalc731ost238
Calculate the mass defect for the formation of phosphorus-31. the mass of a phosphorus-31 nucleus is 30.973765 amu. the masses of a proton and a neutron are 1.00728 and 1.00866 amu, respectively. enter your answer in amu's with five decimal places and no units.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Calculate the mass defect for the formation of phosphorus-31. the mass of a phosphorus-31 nucleus is...
Questions in other subjects:




Geography, 09.03.2020 01:37



Mathematics, 09.03.2020 01:37

Mathematics, 09.03.2020 01:38


Mathematics, 09.03.2020 01:38

![\Delta m=[(n_p\times m_p)+(n_n\times m_n)]-M](/tpl/images/0180/0155/b6e52.png)
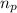 = number of protons
= number of protons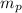 = mass of one proton
= mass of one proton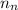 = number of neutrons
= number of neutrons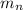 = mass of one neutron
= mass of one neutron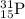
![\Delta m=[(15\times 1.00728)+(16\times 1.00866)]-30.973765\\\\\Delta m=0.27399](/tpl/images/0180/0155/689b8.png)


