Chemistry, 19.08.2019 22:20 RegencySlayer5304
Acertain reaction has an activation energy of 66.41 kj/mol. at what kelvin temperature will the reaction proceed 3.00 times faster than it did at 293 k?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Acertain reaction has an activation energy of 66.41 kj/mol. at what kelvin temperature will the reac...
Questions in other subjects:

Biology, 27.07.2019 06:00


History, 27.07.2019 06:00

History, 27.07.2019 06:00


History, 27.07.2019 06:00


History, 27.07.2019 06:00

History, 27.07.2019 06:00

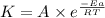
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0179/9186/6d953.png)
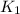 = rate constant at
= rate constant at 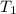 =
= 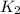 = rate constant at
= rate constant at 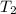 =
= 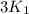
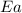 = activation energy for the reaction = 66.41 kJ/mole = 66410 J/mole
= activation energy for the reaction = 66.41 kJ/mole = 66410 J/mole![\log (\frac{3K_1}{K_1})=\frac{66410J/mole}{2.303\times 8.314J/mole.K}[\frac{1}{293K}-\frac{1}{T_2}]](/tpl/images/0179/9186/a03fc.png)