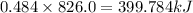Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 23.06.2019 06:20, cowboo5000pcl655
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 09:20, taylorannsalazar
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1

Chemistry, 23.06.2019 10:30, cjtambasco
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1
You know the right answer?
The heat of formation of fe2o3(s) is –826.0 kj/mol. calculate the heat of the reaction 4fe(s) + 3o2(...
Questions in other subjects:



Mathematics, 27.04.2021 07:50

Mathematics, 27.04.2021 07:50



English, 27.04.2021 07:50

Mathematics, 27.04.2021 07:50

Mathematics, 27.04.2021 07:50

English, 27.04.2021 07:50

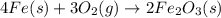
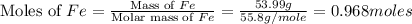
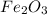
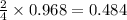 moles of
moles of