

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 11:00, daniel1480
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced. be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
The mean free path of nitrogen molecules at 2.3°c and 1.28 atm is 1.51 x 10-5 cm. at this temperatur...
Questions in other subjects:





Mathematics, 01.04.2021 20:30


Arts, 01.04.2021 20:30


English, 01.04.2021 20:30

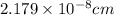 [/tex]
[/tex]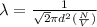
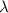 = Mean free path =
= Mean free path = 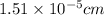
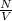 = number of molecules per unit volume =
= number of molecules per unit volume = 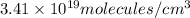
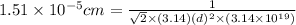
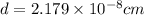 [/tex]
[/tex]

