
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate the molar mass of chloral hydrate. b. what amount (moles) of c2h3cl3o2 molecules are in 500.0 g chloral hydrate? c. what is the mass in grams of 2.0 x 10-2 mol chloral hydrate? d. what number of chlorine atoms are in 5.0 g chloral hydrate? e. what mass of chloral hydrate would contain 1.0 g cl? f. what is the mass of exactly 500 molecules of chloral hydrate?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Chemistry, 23.06.2019 06:30, tdahna0403
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3

Chemistry, 23.06.2019 09:00, hunterwilliams375
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate...
a. calculate...
Questions in other subjects:

Mathematics, 11.10.2019 00:50


Mathematics, 11.10.2019 00:50

Mathematics, 11.10.2019 00:50


Biology, 11.10.2019 00:50

Health, 11.10.2019 00:50


English, 11.10.2019 00:50

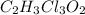 is, 165.5 g/mole
is, 165.5 g/mole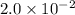 mole chloral hydrate is, 3.31 g
mole chloral hydrate is, 3.31 g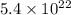

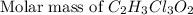 =2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]
=2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]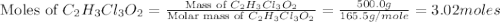
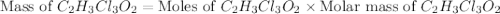
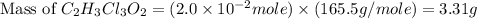
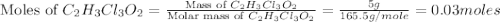
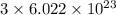 chlorine atoms
chlorine atoms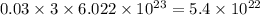 chlorine atoms
chlorine atoms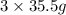 of chlorine present in 165.5 g of
of chlorine present in 165.5 g of 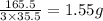 of
of 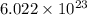 molecules of chloral hydrate has 165.5 g mass of chloral hydrate
molecules of chloral hydrate has 165.5 g mass of chloral hydrate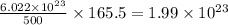 mass of chloral hydrate
mass of chloral hydrate

