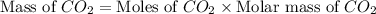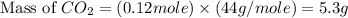Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 1.74 g of butane is mixed with 11. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions in other subjects:

English, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

Arts, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

Biology, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

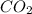 produced will be, 5.3 grams.
produced will be, 5.3 grams.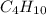 = 1.74 g
= 1.74 g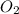 = 11 g
= 11 g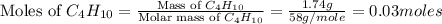
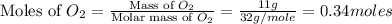
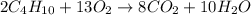
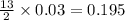 moles of
moles of 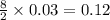 moles of
moles of