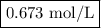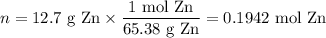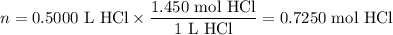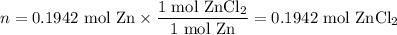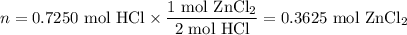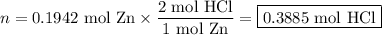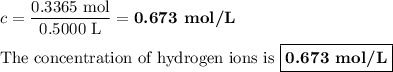Chemistry, 16.08.2019 08:20 JAYDENJONES0111
Zinc dissolves in hydrochloric acid to yield hydrogen gas: zn(s) + 2hcl(aq) --> zncl2(aq) + h2(g) when a 12.7 g chunk of zinc dissolves in 5.00 x 102 ml of 1.450 m hcl, what is the concentration of hydrogen ions remaining in the final solution? 0 m0.388 m0.674 m0.776 m1.06 m

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1


Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Zinc dissolves in hydrochloric acid to yield hydrogen gas: zn(s) + 2hcl(aq) --> zncl2(aq) + h2(...
Questions in other subjects:


Business, 12.11.2019 15:31


English, 12.11.2019 15:31


Social Studies, 12.11.2019 15:31


Social Studies, 12.11.2019 15:31


English, 12.11.2019 15:31