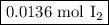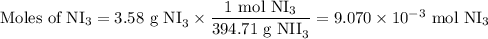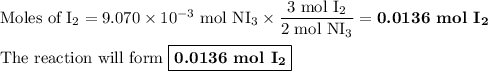Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3



Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
The chemical equation below shows the decomposition of nitrogen triiodide (ni3) into nitrogen (n2) a...
Questions in other subjects:



Business, 04.12.2020 17:00



Mathematics, 04.12.2020 17:00


Mathematics, 04.12.2020 17:00