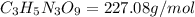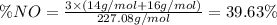Nitrogen monoxide (no) is a bioactive molecule in blood. low no concentrations cause respiratory distress and the formation of blood clots. doctors prescribe nitroglycerin, c3h5n 309. and isoamyl nitrate. (ch3)2chch2ch20n02, to increase no. if each compound releases one molecule of no per atom of n. calculate the mass percent of no in each medicine.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1



Chemistry, 23.06.2019 03:40, ElegantEmerald
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Nitrogen monoxide (no) is a bioactive molecule in blood. low no concentrations cause respiratory dis...
Questions in other subjects:


Mathematics, 08.09.2021 01:00


Mathematics, 08.09.2021 01:00

Mathematics, 08.09.2021 01:00



Mathematics, 08.09.2021 01:00


Biology, 08.09.2021 01:00