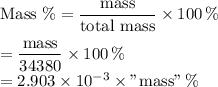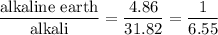The seven most abundant ions in seawater make up more than 99% by mass of the dissolved compounds. here are their abundances in units of mg ion/kg seawater: chloride 18,980; sodium 10,560; sulfate 2650; magnesium 1270; calcium 400; potassium 380; hydrogen carbonate 140. (a) what is the mass 96 of each ion in seawater? (b) what percent of the total mass of ions is sodium ion? (c) how does the total mass % of alkaline earth metal ions compare with the total mass 96 of alkali metal ions? (d) which make up the larger mass fraction of dissolved components, anions or cations?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1


Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 23.06.2019 02:00, xbeatdroperzx
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
The seven most abundant ions in seawater make up more than 99% by mass of the dissolved compounds. h...
Questions in other subjects:

Mathematics, 21.10.2020 20:01

Spanish, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01



Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

English, 21.10.2020 20:01