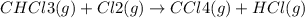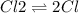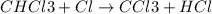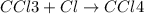Chemistry, 13.08.2019 05:10 meCreation
The reaction chcl3(g) + cl2(g) ccl4(g) + hcl(g) has been proposed to occur by the following mechanism. cl2 2cl fast equilibrium chcl3 + cl hcl + ccl3 slow step ccl3 + cl ccl4 fast what is the rate law predicted by this mechanism? a. rate = k2[chcl3][cl] b. rate = kexp[chcl3][cl2] c. rate = k2(k1/k-1)1/2[chcl3][cl2]1/2 d. rate = k3[ccl3][cl] e. rate = k1[cl2]

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
The reaction chcl3(g) + cl2(g) ccl4(g) + hcl(g) has been proposed to occur by the following mechanis...
Questions in other subjects:



Biology, 12.12.2020 17:00

Physics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Chemistry, 12.12.2020 17:00

Health, 12.12.2020 17:00

Arts, 12.12.2020 17:00