

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2


Chemistry, 23.06.2019 05:30, cluchmasters5634
Scientist think that animals with remarkably similar embryo development probably shared a common ancestor
Answers: 1
You know the right answer?
Write empirical formulas for the following molecules: (a) caffeine (c8h10n4o2), a stimulant found i...
Questions in other subjects:

Social Studies, 05.08.2019 01:30

History, 05.08.2019 01:30

Biology, 05.08.2019 01:30


Health, 05.08.2019 01:30

Chemistry, 05.08.2019 01:30

Mathematics, 05.08.2019 01:30

Mathematics, 05.08.2019 01:30

Mathematics, 05.08.2019 01:30

Biology, 05.08.2019 01:30

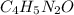
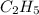
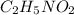
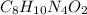 has empirical formula of
has empirical formula of 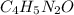
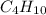 has an empirical formula of
has an empirical formula of 

