
Apossible mechanism for the gas phase reaction of no and h2 is as follows: step 1 2no n2o2 step 2 n2o2 + h2 n2o + h2o step 3 n2o + h2 n2 + h2o which of the following statements concerning this mechanism is not directly supported by the information provided? a. n2o2 is an intermediate. b. all steps are bimolecular reactions. c. the rate expression for step 1 is rate = k[no]2. d. step 1 is the rate determining step. e. there is no catalyst in this reaction. 1 points question 9

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, letsbestupidcx2314
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1


Chemistry, 23.06.2019 01:00, dawnparker71
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
Apossible mechanism for the gas phase reaction of no and h2 is as follows: step 1 2no n2o2 step 2 n...
Questions in other subjects:




Mathematics, 10.04.2020 16:30


Physics, 10.04.2020 16:30

Mathematics, 10.04.2020 16:30

Business, 10.04.2020 16:30


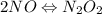 (fast)
(fast)![[NO]^{2}](/tpl/images/0174/7336/f0d3d.png)
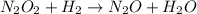
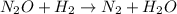 (fast)
(fast)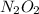 is intermediate in nature.
is intermediate in nature.

