Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, vpowell5371
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 21.06.2019 22:30, connienash95
Explain why scientists use shared characteristics to make cladograms.
Answers: 1


Chemistry, 23.06.2019 01:30, Nathaliasmiles
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
Write the empirical formulas of the following compounds: (a) al2br6, (b) na2s2o4, (c) n2o5, (d) k2c...
Questions in other subjects:


Mathematics, 31.03.2020 21:16





Mathematics, 31.03.2020 21:16

History, 31.03.2020 21:16

History, 31.03.2020 21:16

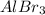
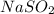
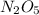
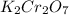
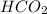
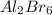 has empirical formula of
has empirical formula of 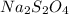 has empirical formula of
has empirical formula of 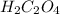 has empirical formula of
has empirical formula of