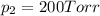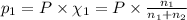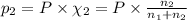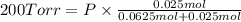Chemistry, 13.08.2019 02:30 lailahussain99
Agas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40.0). if the partial pressure of argon is 200. torr, what is the pressure of methane, in torr? hint: what is the mole fraction of each gas?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
Agas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40...
Questions in other subjects:



Biology, 12.07.2019 12:00

Biology, 12.07.2019 12:00




Health, 12.07.2019 12:00

Arts, 12.07.2019 12:00

Mathematics, 12.07.2019 12:00

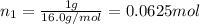
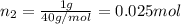
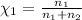
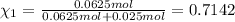
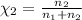
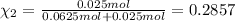
 .
.