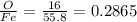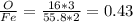Chemistry, 13.08.2019 01:30 katwright1124
Both feo and fe2o3 contain only iron and oxygen. the mass ratio of oxygen to iron for each compound is given in the following table:
compound mass o : mass fe
feo 0.2865
fe2o3 0.4297
show that these data are consistent with the law of multiple proportions.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1


Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
Both feo and fe2o3 contain only iron and oxygen. the mass ratio of oxygen to iron for each compound...
Questions in other subjects:

Mathematics, 03.04.2021 14:03


Computers and Technology, 03.04.2021 14:03

Mathematics, 03.04.2021 14:03



Computers and Technology, 03.04.2021 14:03

Chemistry, 03.04.2021 14:03


Chemistry, 03.04.2021 14:03