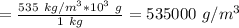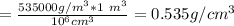Chemistry, 13.08.2019 00:30 Chandler1Gaming
The density of lithium metal is 535 kg/m3. what is this density in g/cm3? a) 0.000535 g/cm^3 b) 0.535 g/cm^3 c) 0.0535 g/cm^3 d) 0.54 g/cm^3 e) 53.5 g/cm^3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
The density of lithium metal is 535 kg/m3. what is this density in g/cm3? a) 0.000535 g/cm^3 b) 0.5...
Questions in other subjects:



Mathematics, 19.03.2021 19:40



Mathematics, 19.03.2021 19:40


History, 19.03.2021 19:40

Mathematics, 19.03.2021 19:40