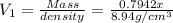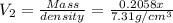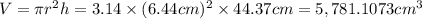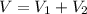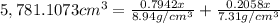Chemistry, 12.08.2019 23:30 Dragonskeld
Bronze is an alloy made of copper (cu) and tin (sn). calculate the mass of a bronze cylinder of radius 6.44 cm and length 44.37 cm. the composition of the bronze is 79.42 percent cu and 20.58 percent sn and the densities of cu and sn are 8.94 g/cm^3 and 7.31 g/cm^3, respectively. what assumption should you make in this calculation?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, BigDough9090
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 08:30, vanessadaniellet21
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
Bronze is an alloy made of copper (cu) and tin (sn). calculate the mass of a bronze cylinder of radi...
Questions in other subjects:

Chemistry, 21.11.2020 23:40

Engineering, 21.11.2020 23:40

Social Studies, 21.11.2020 23:40

Mathematics, 21.11.2020 23:40




Social Studies, 21.11.2020 23:40


Mathematics, 21.11.2020 23:40