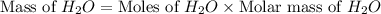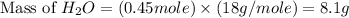Chemistry, 12.08.2019 23:30 nmartin5185
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
ch4 (g) + 2 o2 (g) --> co2 (g) + 2 h2o (l)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, falishaduncanovmtz2
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3



Chemistry, 23.06.2019 02:00, xbeatdroperzx
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
Questions in other subjects:




Mathematics, 13.05.2021 21:50

Business, 13.05.2021 21:50



Mathematics, 13.05.2021 21:50


Geography, 13.05.2021 21:50

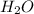 produced will be, 8.1 grams.
produced will be, 8.1 grams.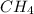 =
= 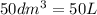
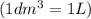
 =
= 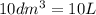
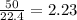 mole of
mole of 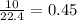 mole of
mole of 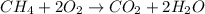
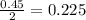 moles of
moles of