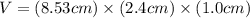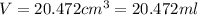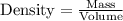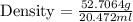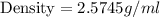Chemistry, 12.08.2019 22:10 andrewmena05
In determining the density of a rectangular metal bar, a student made the following measurements: length, 8.53 cm; width, 2.4 cm; height, 1.0 cm; mass, 52.7064 g. calculate the density of the metal to the correct number of significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 01:30, emfranco1
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
In determining the density of a rectangular metal bar, a student made the following measurements: l...
Questions in other subjects:

Computers and Technology, 07.10.2020 14:01



History, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

History, 07.10.2020 14:01



English, 07.10.2020 14:01