
Chemistry, 12.08.2019 21:30 rhyanebean6443
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts with oxygen gas, producing carbon dioxide gas and water vapor. calculate the moles of carbon dioxide produced by the reaction of 1.5 mol of oxygen. be sure your answer has a unit symbol, if necessary, and round it to significant digits.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 10:00, 2019reynolds
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1
You know the right answer?
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts...
Questions in other subjects:




Business, 28.01.2020 02:31


Mathematics, 28.01.2020 02:31




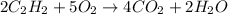
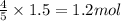 of carbon dioxide.
of carbon dioxide.

