
Chemistry, 12.08.2019 21:20 edfrank6278
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.500 m solution of aspirin has a ph of 1.86. you are interested in learning about the % dissociation in a buffered solution of aspirin, so you make a new 1.00 l solution containing 0.500 moles of aspirin and 0.25 moles of the sodium salt of aspirin. what will the % dissociation be in this new buffered solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, salvadorperez26
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the value of ksp for caf2 at 25°c is 4.0 x 10-11. will this solution form a precipitate? yes no
Answers: 3

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 15:30, vivianfling
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.50...
Questions in other subjects:

Mathematics, 30.10.2020 16:10


Social Studies, 30.10.2020 16:10

Mathematics, 30.10.2020 16:10



Mathematics, 30.10.2020 16:10

History, 30.10.2020 16:10

History, 30.10.2020 16:10

Mathematics, 30.10.2020 16:10

![-log[H^{+}]](/tpl/images/0174/4805/1d5a1.png)
![[H^{+}] = 10^{-pH}](/tpl/images/0174/4805/241df.png)
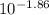
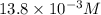
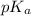 value will be calculated as follows.
value will be calculated as follows.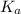 =
= ![\frac{[H^{+}]^{2}}{[Aspirin]}](/tpl/images/0174/4805/0efaa.png)
![\frac{[13.8 \times 10^{-3}]^{2}}{0.50}](/tpl/images/0174/4805/d6d3a.png)
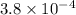
![pK_{a} = -log [K_{a}]](/tpl/images/0174/4805/95c79.png)
![-log [3.8 \times 10^{-4}]](/tpl/images/0174/4805/46d55.png)
![pK_{a} + log \frac{[CH_{3}COO^{-}]}{[Aspirin]}](/tpl/images/0174/4805/cc4ea.png)
![log \frac{[0.25]}{[0.5]}](/tpl/images/0174/4805/52faa.png)
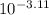
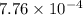 M
M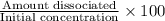
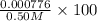
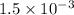 × 100
× 100

