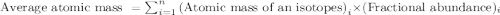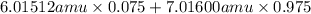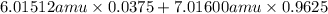Chemistry, 12.08.2019 19:20 kaywendel2008
Variations in average atomic mass may be observed for elements obtained from different sources. lithium provides an example of this. the isotopic composition of lithium from naturally occurring minerals is 7.5% 6li and 92.5% 7li, which have masses of 6.01512 amu and 7.01600 amu, respectively. a commercial source of lithium, recycled from a military source, was 3.75% 6li (and the rest 7li). calculate the average atomic mass values for each of these two sources.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 23.06.2019 09:40, 23rwilliamson
Is cutting your nails a physical or chemical change
Answers: 2

You know the right answer?
Variations in average atomic mass may be observed for elements obtained from different sources. lith...
Questions in other subjects:


Social Studies, 15.01.2020 04:31



Biology, 15.01.2020 04:31

Mathematics, 15.01.2020 04:31

Chemistry, 15.01.2020 04:31

Arts, 15.01.2020 04:31


Mathematics, 15.01.2020 04:31