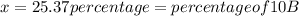The average atomic masses of some elements may vary, depending upon souces of their ores. naturally occurring boron consist of two isotopes with accurately known masses (10 b, 10.0129 amu and 11b, 11.0931 amu). the actual atomic mass of boron can vary grom 10.807 to 10819, depending on whether the mineral source is from turkey or the united states. calculate the percent abundances leading to the two values of the average atomic masses of boron from these two countries.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
The average atomic masses of some elements may vary, depending upon souces of their ores. naturally...
Questions in other subjects:


Arts, 06.10.2021 02:00

Mathematics, 06.10.2021 02:00


Social Studies, 06.10.2021 02:00

Mathematics, 06.10.2021 02:00

Mathematics, 06.10.2021 02:00

Mathematics, 06.10.2021 02:00


![\frac{[percentageofisotope(1)Xatomicmassofisotope(1)]+[percentageofisotope(2)Xatomicmassofisotope(2)}{100}](/tpl/images/0174/3972/b6fa0.png)
![10.807=\frac{[x(10.0129)]+[(100-x)11.0931]}{100}=\frac{10.0129x+1109.31-11.0931x}{100}](/tpl/images/0174/3972/4e3aa.png)
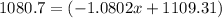

![10.819=\frac{[x(10.0129)]+[(100-x)11.0931]}{100}=\frac{10.0129x+1109.31-11.0931x}{100}](/tpl/images/0174/3972/7b5b5.png)