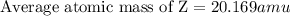Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
An element has the following natural abundances and isotopic masses: 90.92% abundance with 19.99 am...
Questions in other subjects:








Business, 24.01.2020 03:31


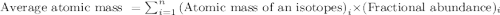 .....(1)
.....(1)![\text{Average atomic mass of Z}=[(19.99\times 0.9092)+(20.99\times 0.0026)+(21.99\times 0.0882)]](/tpl/images/0174/3907/323be.png)