
Chemistry, 12.08.2019 18:30 briarkaltvedt
Calculate δ h° for the reaction c 4h 4( g) + 2h 2( g) → c 4h 8( g), using the following data: δ h° combustion for c 4h 4( g) = –2341 kj/mol δ h° combustion for h 2( g) = –286 kj/mol δ h° combustion for c 4h 8( g) = –2755 kj/mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

You know the right answer?
Calculate δ h° for the reaction c 4h 4( g) + 2h 2( g) → c 4h 8( g), using the following data: δ h°...
Questions in other subjects:


Mathematics, 22.01.2021 07:30


Arts, 22.01.2021 07:30



History, 22.01.2021 07:30



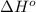
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_{(product)}]-\sum [n\times \Delta H^o_{(reactant)}]](/tpl/images/0174/3643/6872e.png)
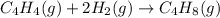
![\Delta H^o_{rxn}=[(1\times \Delta H^o_{(C_4H_8(g))})]-[(1\times \Delta H^o_{(C_4H_4(g))})+(2\times \Delta H^o_{(H_2(g))})]](/tpl/images/0174/3643/605f9.png)
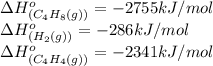
![\Delta H^o_{rxn}=[(1\times (-2755))]-[(1\times (-286))+(2\times (-2341))]\\\\\Delta H^o_{rxn}=2213kJ](/tpl/images/0174/3643/044fa.png)


