
Chemistry, 12.08.2019 17:10 silveryflight
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3). the balanced equation isc7h6o3 + c4h6o3 → c9h8o4 + hc2h3o2(a) what mass of acetic anhydride is needed to completely consume 1.00 x 10^2 g salicylic acid? (b) what is the maximum mass of aspirin (the theoretical yield) that could be produced in this reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, leannamat2106
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 21.06.2019 19:00, RedDemon59
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2


Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3
You know the right answer?
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3)....
Questions in other subjects:




History, 16.10.2019 20:00



Mathematics, 16.10.2019 20:00


Biology, 16.10.2019 20:00

Physics, 16.10.2019 20:00

 .....(1)
.....(1)

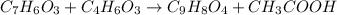
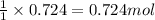 of acetic anhydride.
of acetic anhydride.




