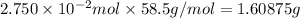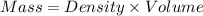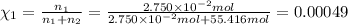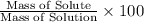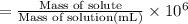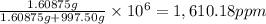Chemistry, 12.08.2019 16:10 lyndamahe0
A2.750×10−2m solution of nacl in water is at 20.0∘c. the sample was created by dissolving a sample of nacl in water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 999.3 ml . the density of water at 20.0∘c is 0.9982 g/ml.
calculate the mole fraction of salt in this solution.
calculate the concentration of the salt solution in percent by mass.
calculate the concentration of the salt solution in parts per million.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
A2.750×10−2m solution of nacl in water is at 20.0∘c. the sample was created by dissolving a sample o...
Questions in other subjects:


Mathematics, 22.06.2019 19:30

Mathematics, 22.06.2019 19:30

English, 22.06.2019 19:30

Mathematics, 22.06.2019 19:30



Mathematics, 22.06.2019 19:30


Mathematics, 22.06.2019 19:30


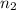
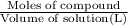


 of NaCl :
of NaCl :