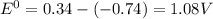Agalvanic (voltaic) cell consists of an electrode composed of chromium in a 1.0 m chromium(iii) ion solution and another electrode composed of copper in a 1.0 m copper(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25 °c. refer to the list

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, notkeandre9
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
Agalvanic (voltaic) cell consists of an electrode composed of chromium in a 1.0 m chromium(iii) ion...
Questions in other subjects:


English, 27.05.2020 05:59



Mathematics, 27.05.2020 05:59


Mathematics, 27.05.2020 05:59

Mathematics, 27.05.2020 05:59


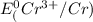 =-0.74V[/tex]
=-0.74V[/tex]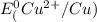 =0.34V[/tex]
=0.34V[/tex]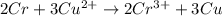
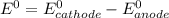
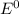 are standard reduction potentials, when concentration is 1M.
are standard reduction potentials, when concentration is 1M.![E^0=E^0_{[Cu^{2+}/Ni]}- E^0_{[Cr^{3+}/Cr]}](/tpl/images/0174/1025/838bc.png)