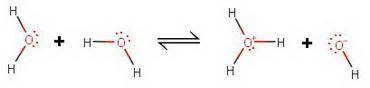Chemistry, 10.08.2019 01:30 rickevaaaa
Complete the equation for the ionization of water by drawing the conjugate acid and conjugate base. include lone pairs of electrons. two water molecules react to form the conjugate acid which has a plus 1 charge, and the conjugate base, which has a minus one charge. each water molecule consists of a central oxygen atom bonded to two hydrogen atoms. there are two lone pairs on the oxygen atom.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Garciaapril1597
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
Complete the equation for the ionization of water by drawing the conjugate acid and conjugate base....
Questions in other subjects:



Mathematics, 29.04.2021 01:10






Mathematics, 29.04.2021 01:10

Mathematics, 29.04.2021 01:10