
Suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with iron(ii) chloride, which would react with silver nitrate solution like this: fecl2 (aq) + 2agno3 (aq) → 2agcl (s) + feno32 (aq) the chemist adds 14.0m m silver nitrate solution to the sample until silver chloride stops forming. she then washes, dries, and weighs the precipitate. she finds she has collected 6.9mg of silver chloride. calculate the concentration of iron(ii) chloride contaminant in the original groundwater sample. be sure your answer has the correct number of significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3


Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
Suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with iron(ii) c...
Questions in other subjects:


Mathematics, 24.03.2021 19:50




Chemistry, 24.03.2021 19:50

Biology, 24.03.2021 19:50

Biology, 24.03.2021 19:50


Mathematics, 24.03.2021 19:50

 .
.
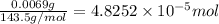
 moles of silver chloride will be obtained from:
moles of silver chloride will be obtained from:

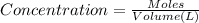
![[FeCl_2]=\frac{2.4126\times 10^{-5} mol}{0.2 L}=0.00012063 mol/L](/tpl/images/0173/9764/69c0f.png)
![[FeCl_2]=1.2063\times 10^{-4} mol/L\approx 1.2\times 10^{-4} mol/L](/tpl/images/0173/9764/77e47.png)


