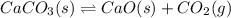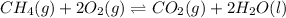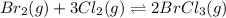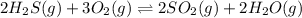Chemistry, 10.08.2019 00:10 dheydar3506
Each of the following reactions is allowed to reach equilibrium in a sealed container. for which of the reactions could you shift the equilibrium to the right by decreasing the pressure?
ch4(g) + 2o2(g) double arrow yields co2(g) + 2h2o(l)
caco3(s) double arrow yields cao(s) + co2(g)
br2(g) + 3cl2(g) double arrow yields 2brcl3(g)
2h2s(g) + 3o2(g) double arrow yields 2so2(g) + 2h2o(g)

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:00, hellodarkness14
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
You know the right answer?
Each of the following reactions is allowed to reach equilibrium in a sealed container. for which of...
Questions in other subjects:


English, 10.06.2020 06:57






History, 10.06.2020 06:57