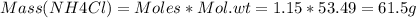Chemistry, 09.08.2019 23:30 rosanaboyd7
Calculate the number of grams of ammonium chloride that must be added to 2.00 l of a 0.500 m ammonia solution to obtain a buffer of ph = 9.20. assume the volume of the solution does not change as the solid is added. kb for ammonia is 1.80×10−5.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1


Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3
You know the right answer?
Calculate the number of grams of ammonium chloride that must be added to 2.00 l of a 0.500 m ammonia...
Questions in other subjects:

Mathematics, 28.08.2019 13:50

English, 28.08.2019 13:50



English, 28.08.2019 13:50


Mathematics, 28.08.2019 13:50


Mathematics, 28.08.2019 13:50

Physics, 28.08.2019 13:50

![pOH = pKb + log\frac{[BH+]}{[B]}](/tpl/images/0173/9475/1dae4.png)

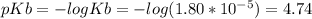
![4.8 = 4.74 + log\frac{[NH4Cl]}{[0.500]}](/tpl/images/0173/9475/4bd8c.png)