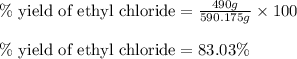Chemistry, 09.08.2019 23:20 4804397217
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calculate the percent yield of c2h5cl if the reaction of 300 g of ethane with 650 g of chlorine produced 490 g of c2h5cl .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, markmlg122
Two things that biomedical has invented or innvated
Answers: 1

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

You know the right answer?
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calcu...
Questions in other subjects:

Mathematics, 04.09.2021 20:10



Mathematics, 04.09.2021 20:10





Health, 04.09.2021 20:20

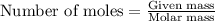 ....(1)
....(1)
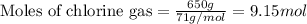

 of ethane gas.
of ethane gas.