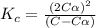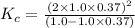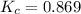Chemistry, 09.08.2019 19:20 pwolfiimp4
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibrium described by the equation n2o4(g) ↔ 2no2(g) if at equilibrium the n2o4 is 37% dissociated, what is the value of the equilibrium constant, kc, for the reaction under these conditions?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lukeakalucas
Alarge marble is dropped in a graduated cylinder with 35ml of water in it. the water level increases to 49ml. what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1
You know the right answer?
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibrium descr...
Questions in other subjects:

History, 13.07.2019 17:40


Mathematics, 13.07.2019 17:40

Biology, 13.07.2019 17:40






Social Studies, 13.07.2019 17:40

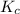 for the reaction is, 0.869
for the reaction is, 0.869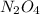 .
.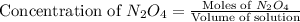
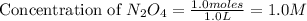
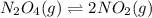
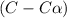
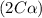
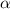 = 37 % = 0.37
= 37 % = 0.37![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0173/7935/271f5.png)