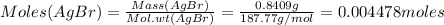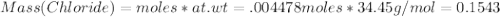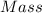Amixture of crbr3 and inert material is analyzed to determine the cr content. first the mixture is dissolved in water. then all of the bromide ion is precipitated as agbr by the addition of an excess of silver nitrate. crbr3(aq) + 3agno3(aq) 3agbr(s) + cr(no3)3(aq) in one experiment, a 0.8409 g sample of the mixture resulted in 1.0638 g of agbr. determine the percent (by mass) of cr in the mixture.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, daigle18383
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3


Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Amixture of crbr3 and inert material is analyzed to determine the cr content. first the mixture is d...
Questions in other subjects:

Chemistry, 02.02.2020 07:43

Mathematics, 02.02.2020 07:43

Mathematics, 02.02.2020 07:43

Mathematics, 02.02.2020 07:44


English, 02.02.2020 07:44

Biology, 02.02.2020 07:44

Physics, 02.02.2020 07:44


Physics, 02.02.2020 07:44