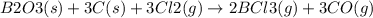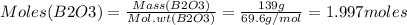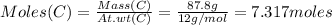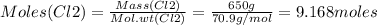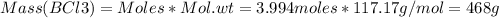Consider the reaction of diboron trioxide with carbon and chlorine. b2o3 (s) + 3c (s) + 3cl2 (g) 2bcl3 (g) + 3co (g) determine the limiting reactant in a mixture containing 139 g of b2o3, 87.8 g of c, and 650 g of cl2. calculate the maximum mass (in grams) of boron trichloride, bcl3, that can be produced in the reaction. the limiting reactant is: b2o3 cl2 c amount of bcl3 formed = g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
Consider the reaction of diboron trioxide with carbon and chlorine. b2o3 (s) + 3c (s) + 3cl2 (g) 2bc...
Questions in other subjects:

Mathematics, 20.05.2020 01:57

Mathematics, 20.05.2020 01:57

Mathematics, 20.05.2020 01:57

History, 20.05.2020 01:57

Mathematics, 20.05.2020 01:57

Business, 20.05.2020 01:57

Mathematics, 20.05.2020 01:57

Mathematics, 20.05.2020 01:57

Geography, 20.05.2020 01:57

Mathematics, 20.05.2020 01:57