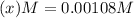Chemistry, 08.08.2019 06:20 baeethtsadia
At a particular temperature, k 5.0 x 10-6 for the reaction 2co2(9) 2co(9) +02(9) if 3.0 moles of co2 is initially placed into a 5.0-l vessel, calculate the equilibrium concentrations of all species. co2] co] o2l [02]-

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, ayoismeisalex
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2


Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 08:00, ira51
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
At a particular temperature, k 5.0 x 10-6 for the reaction 2co2(9) 2co(9) +02(9) if 3.0 moles of co2...
Questions in other subjects:










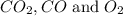 at equilibrium will be, 0.598 M, 0.00216 M and 0.00108 M respectively.
at equilibrium will be, 0.598 M, 0.00216 M and 0.00108 M respectively.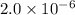
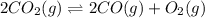
![K_c=\frac{[CO]^2[O_2]}{[CO_2]^2}](/tpl/images/0173/1740/96a03.png)
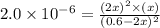
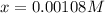
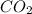 at equilibrium =
at equilibrium = ![(0.6-2x)M=[0.6-2(0.00108)]M=0.598M](/tpl/images/0173/1740/7a4da.png)
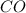 at equilibrium =
at equilibrium = ![(2x)M=[2(0.00108)]M=0.00216M](/tpl/images/0173/1740/eff4f.png)
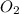 at equilibrium =
at equilibrium =