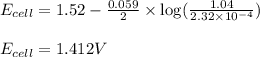Chemistry, 08.08.2019 06:10 david1236544
what is the calculated value of the cell potential at 298k for an electrochemical cell with the following reaction, when the cu2+ concentration is 2.32×10-4 m and the mn2+ concentration is 1.04 m ?
cu2+(aq) + mn(s) cu(s) + mn2+(aq)
the cell reaction as written above is spontaneous for the concentrations given: (true/false)

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 22:00, Porciabeauty6788
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
what is the calculated value of the cell potential at 298k for an electrochemical cell with the foll...
Questions in other subjects:


Advanced Placement (AP), 03.03.2021 06:50

Chemistry, 03.03.2021 06:50

Mathematics, 03.03.2021 06:50

Mathematics, 03.03.2021 06:50





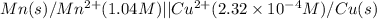
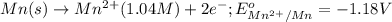
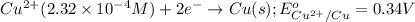

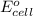 of the reaction, we use the equation:
of the reaction, we use the equation: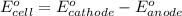
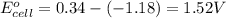
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]}{[Cu^{2+}]}](/tpl/images/0173/1697/70482.png)
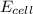 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[Cu^{2+}]=2.32\times 10^{-4}M](/tpl/images/0173/1697/f12ce.png)
![[Mn^{2+}]=1.04M](/tpl/images/0173/1697/250ae.png)