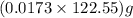8. a sample of potassium chlorate (kcio,) was heated in a test tube and decomposed 2kc? (s) 302 (g) + 2kcio, (s) the oxygen was collected by the displacement of water at 22'c at a total pressure of 754 torr. the volume of the gas collected was 0.65l and the vapor pressure of water at 22°c is 21 torr. calculate a) the partial pressure of o2 in the gas collected and b) the mass of kcio3 in the sample that was decomposed

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, miamassimino
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
8. a sample of potassium chlorate (kcio,) was heated in a test tube and decomposed 2kc? (s) 302 (g)...
Questions in other subjects:

Business, 11.02.2021 19:50


Mathematics, 11.02.2021 19:50

Mathematics, 11.02.2021 19:50

English, 11.02.2021 19:50


Spanish, 11.02.2021 19:50


Mathematics, 11.02.2021 19:50

Mathematics, 11.02.2021 19:50

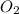 in the gas was 733 torr and mass of
in the gas was 733 torr and mass of 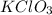 in the sample was 2.12 g.
in the sample was 2.12 g.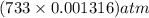 = 0.9646 atm
= 0.9646 atm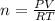
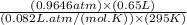
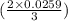 moles or 0.0173 moles of
moles or 0.0173 moles of