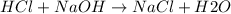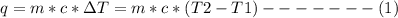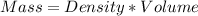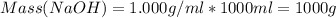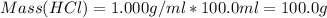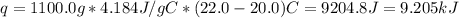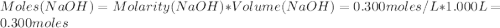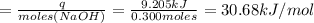Chemistry, 08.08.2019 06:10 joshuahinton45
A1000 ml sample of 0.300 m naoh is mixed with 100.0 ml of 0.300 m hcl in a coffee cup calorimeter. if both solutions are at 20.0 °c and the final temperature of the mixture was 22.0 °c find the heat of neutralization, ahreu in kj/mole. assume no heat is lost to the surroundings, the density of all solutions is 1.00 g/ml, and c, of the mixture is 4.184 j/g·°c

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 01:30, Michael845313
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 09:20, annapittbull12
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2

Chemistry, 23.06.2019 12:00, cooltrey777
How far in advance is weather forecasting most accurate
Answers: 2
You know the right answer?
A1000 ml sample of 0.300 m naoh is mixed with 100.0 ml of 0.300 m hcl in a coffee cup calorimeter. i...
Questions in other subjects:

Mathematics, 18.02.2020 02:42





English, 18.02.2020 02:43