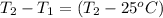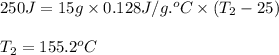Chemistry, 08.08.2019 05:30 jhashknkughb6759
A15.0 g metal sample at 25.0 °c has 250 j of heat added to it. the specific heat of the metal is 0.128 j/g.°c. what is the final temperature?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2
You know the right answer?
A15.0 g metal sample at 25.0 °c has 250 j of heat added to it. the specific heat of the metal is 0.1...
Questions in other subjects:


Mathematics, 04.07.2019 03:30




History, 04.07.2019 03:30


English, 04.07.2019 03:30

Mathematics, 04.07.2019 03:30

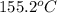
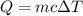
 = change in temperature =
= change in temperature =