Chemistry, 08.08.2019 05:30 katier9407
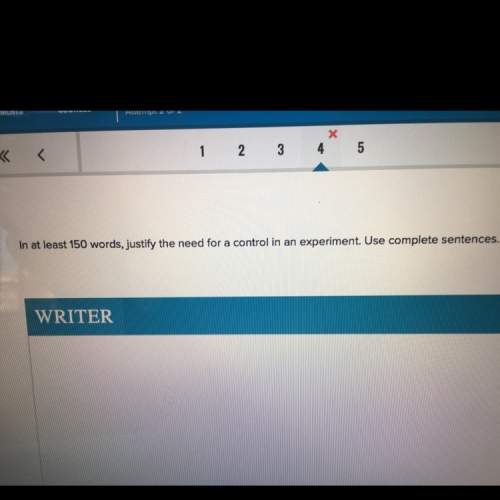
Nernst equation – for the following reaction:
mn (s) + cr3+ (aq) ? mn2+ (aq) + cr (s)
(a) write the three balanced equations (each half-cell and the overall):
(b) what is the standard cell potential (e°cell) for this mn/cr cell?
(c) what will be the cell potential when [mn2+] = 0.20 m and [cr3+] = 1.0 × 10-4 m ?
(d) what will be the cell potential when [mn2+] = 1.0 × 10-4 m and [cr3+] = 0.20 m ?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1


Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1
You know the right answer?
Nernst equation – for the following reaction:
mn (s) + cr3+ (aq) ? mn2+ (aq) + cr (s)
...
mn (s) + cr3+ (aq) ? mn2+ (aq) + cr (s)
...
Questions in other subjects:


Mathematics, 29.10.2020 23:20





Mathematics, 29.10.2020 23:20


Mathematics, 29.10.2020 23:20

Mathematics, 29.10.2020 23:20

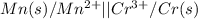
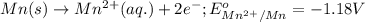 ( × 3)
( × 3)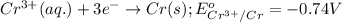 ( × 2)
( × 2)
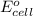 of the reaction, we use the equation:
of the reaction, we use the equation: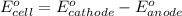

![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]}{[Cr^{3+}]}](/tpl/images/0173/1570/b6936.png)
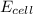 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[Cr^{3+}]=1.0\times 10^{-4}M](/tpl/images/0173/1570/252be.png)
![[Mn^{2+}]=0.20M](/tpl/images/0173/1570/1716f.png)
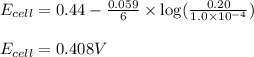
![[Cr^{3+}]=0.20M](/tpl/images/0173/1570/cae64.png)
![[Mn^{2+}]=1.0\times 10^{-4}M](/tpl/images/0173/1570/83dbd.png)